What is an artificial leaf?
Photosynthesis and its working
Most of the scientific advancements and strides “Man” has made, come directly from observing and studying nature. It is as if nature presents the questions and contains the answers within itself, which are just needed to be explored. This fact can be better understood with countless examples, the most prominent of which will be making airplanes by watching the flight of birds, or diving equipment by observing the structure and nature of fish and underwater creatures. Similarly, the idea of generating fuel through photochemical reactions was coined by Giacomo Ciamician1, which was based on the photochemical reactions occurring during the photosynthesis of plants. Therefore, in order to understand the physics and almost the whole working principle of artificial leaves, which can generate energy, we need to understand photosynthesis in a bit more detail. We have tried to present the general concept here without delving into too much chemistry.
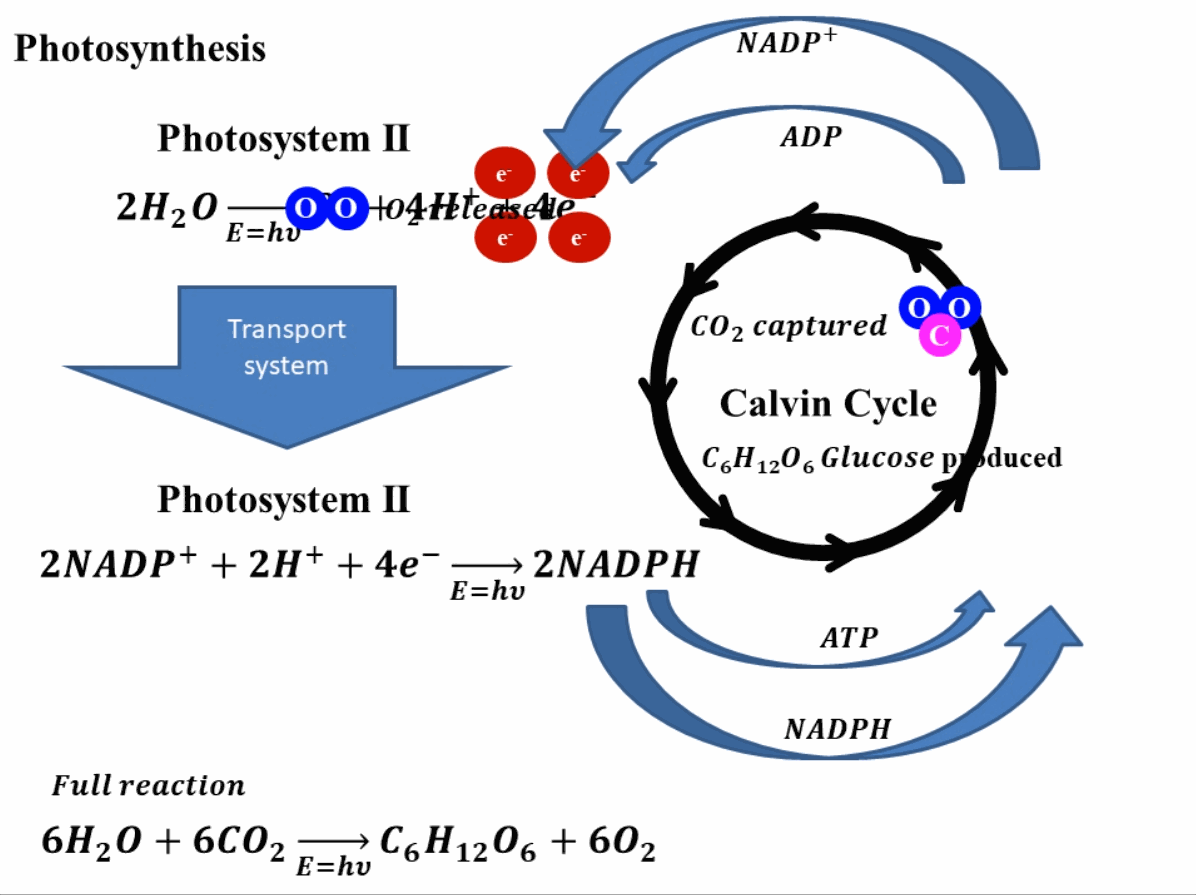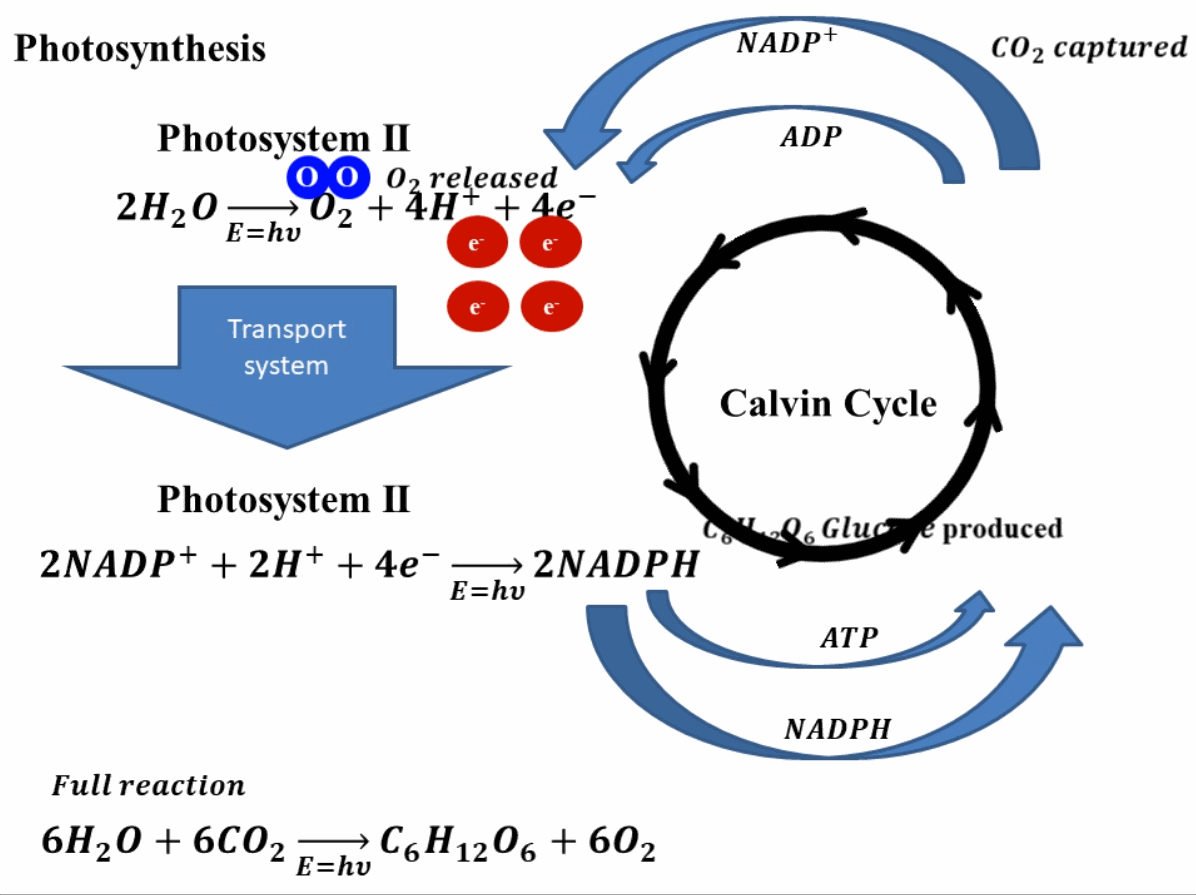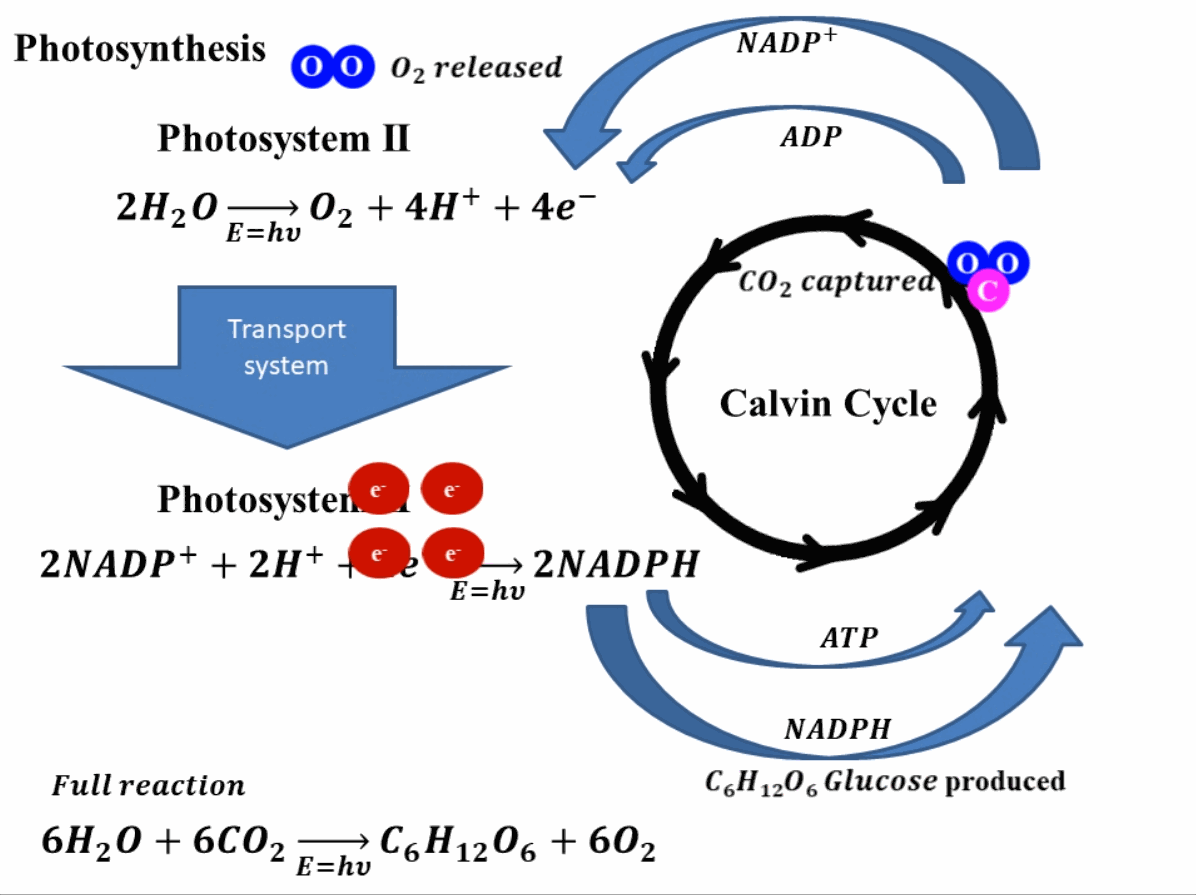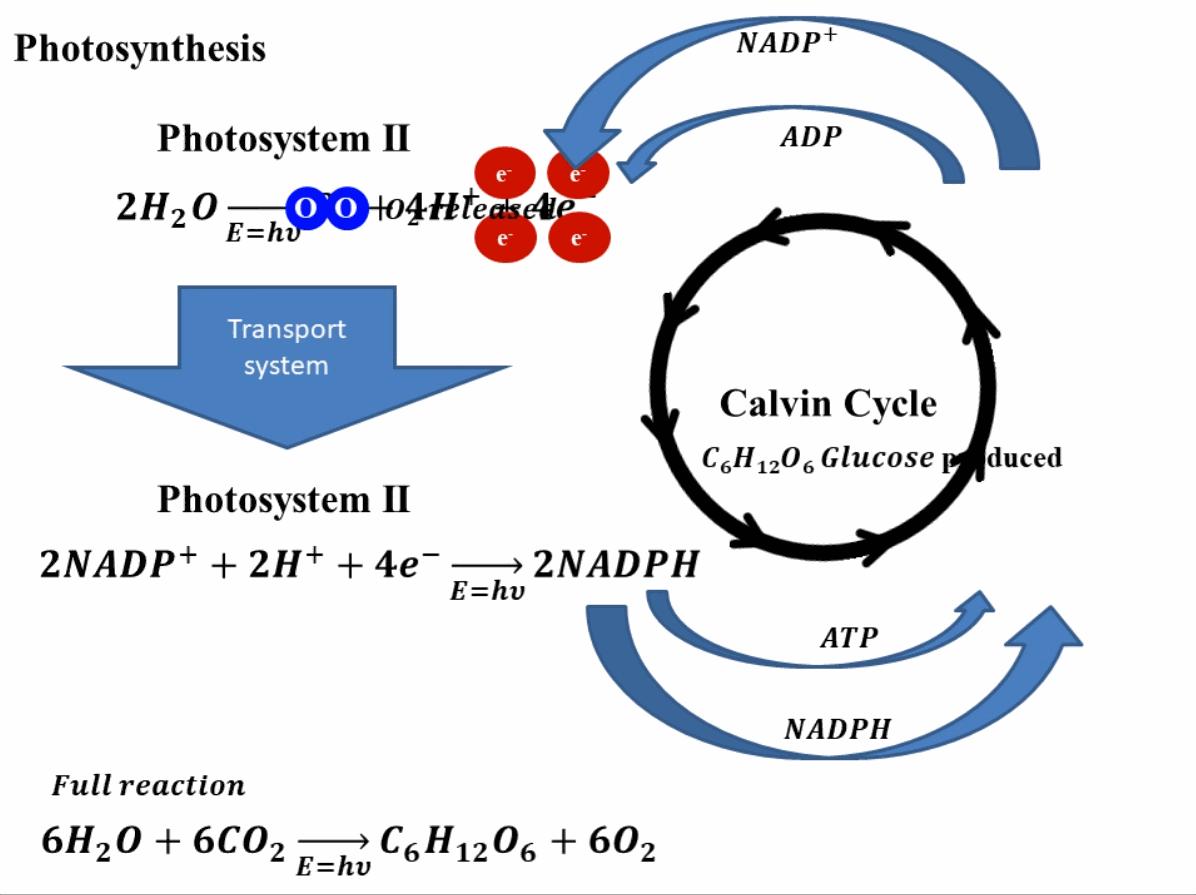
Photosynthesis starts by absorbing solar energy, or in planar terms, light. This light is absorbed in photosystem II of the plant. Through this absorption of light, the water molecules break down into hydrogen ions (or protons) and oxygen molecules. The oxygen produces is released into the environment and this is how the green leaves produce oxygen. Apart from hydrogen ions and oxygen, this reaction also produces four electrons. These electrons go through a transport system, finally reaching photosystem I2. In photosystem I, these electrons reduce nicotinamide adenine dinucleotide phosphate (NADP+) which combines with hydrogen ions or protons to produce NADPH. In this stage, adenosine diphosphate (ADP) is also reduced to ATP (adenosine triphosphate). The produced NADPH and ATP, reduce the carbon dioxide (CO2) into sugar such as glucose (C6H12O6). The reduction of NADP+ and ADP to NADPH and ATP and vice versa is a continuous process and is called the Calvin cycle. A depiction of this whole process is shown in Fig. 1 for better understanding.
Artificial leaf
Just like photosynthesis, scientists are trying to develop these reactions occurring in photosystem II and photosystem I artificially. The reactions are similar to photosynthesis in which oxygen is produced in one reaction in presence of the catalysts which evolve (emit) oxygen3 (OCE-Oxygen Evolving Catalyst) by breaking down water. In the second reaction, the Proton-Coupled Electron Transfer (PCET) catalyst is present which reduces the hydrogen ions (or protons) to evolve hydrogen. This step produces hydrocarbons, which can be used as fuels. In reality, when these hydrocarbons are used as fuel, they take absorb oxygen and release carbon dioxide into the air, eventually making the process of obtaining energy from the artificial leaf a carbon-neutral process (does not add to the effect of global warming that causes climate change). A depiction of an artificial leaf system is provided in Fig. 2. Many different materials are explored in developing artificial leaf systems with various catalysts, therefore, only important terminologies are mentioned, whereas, the basic operation of artificial leaf remains the same.
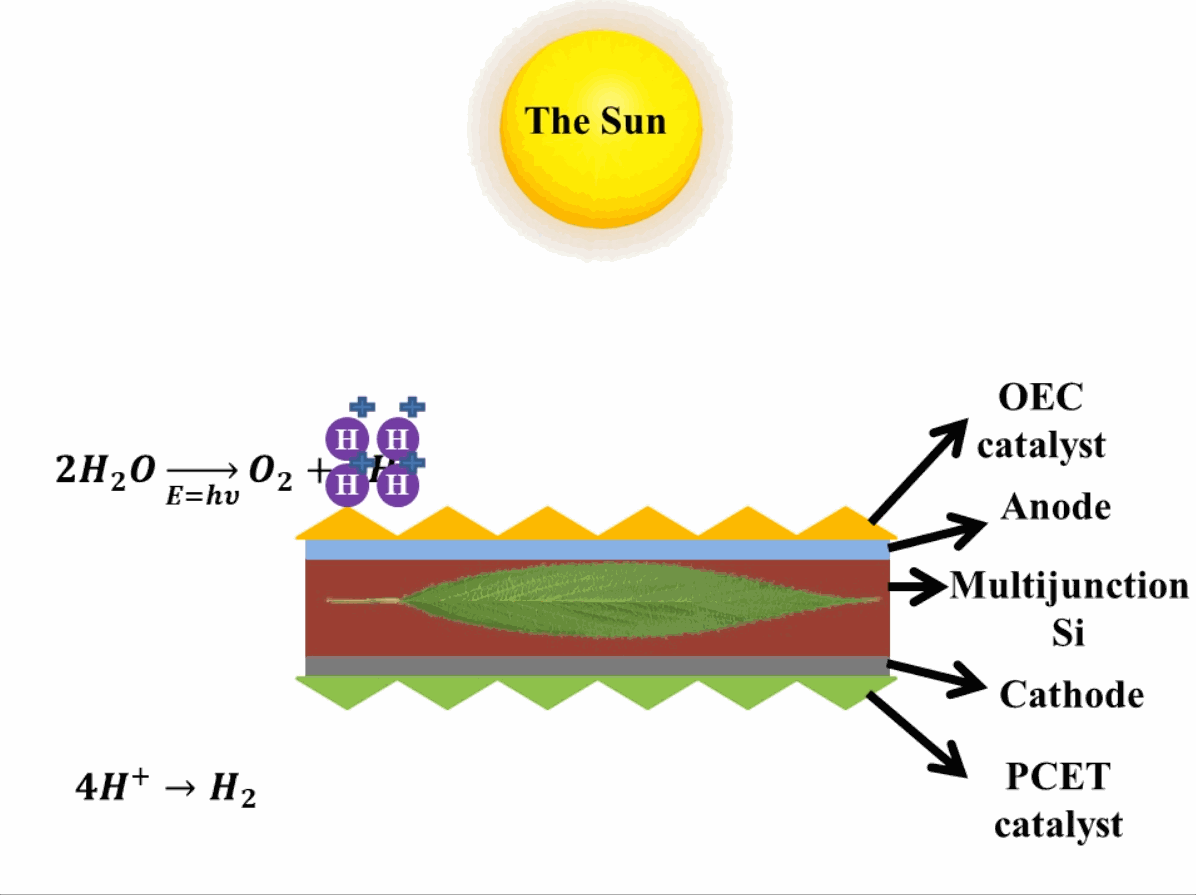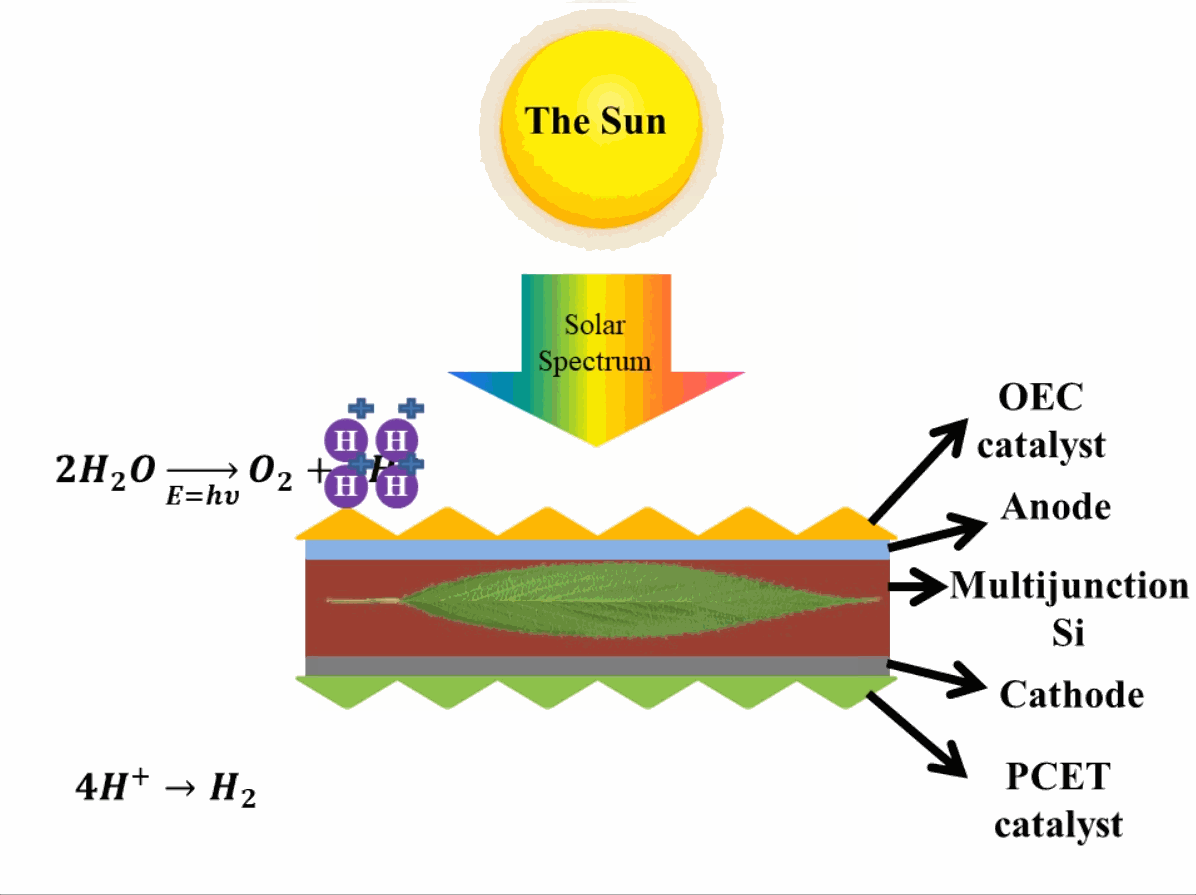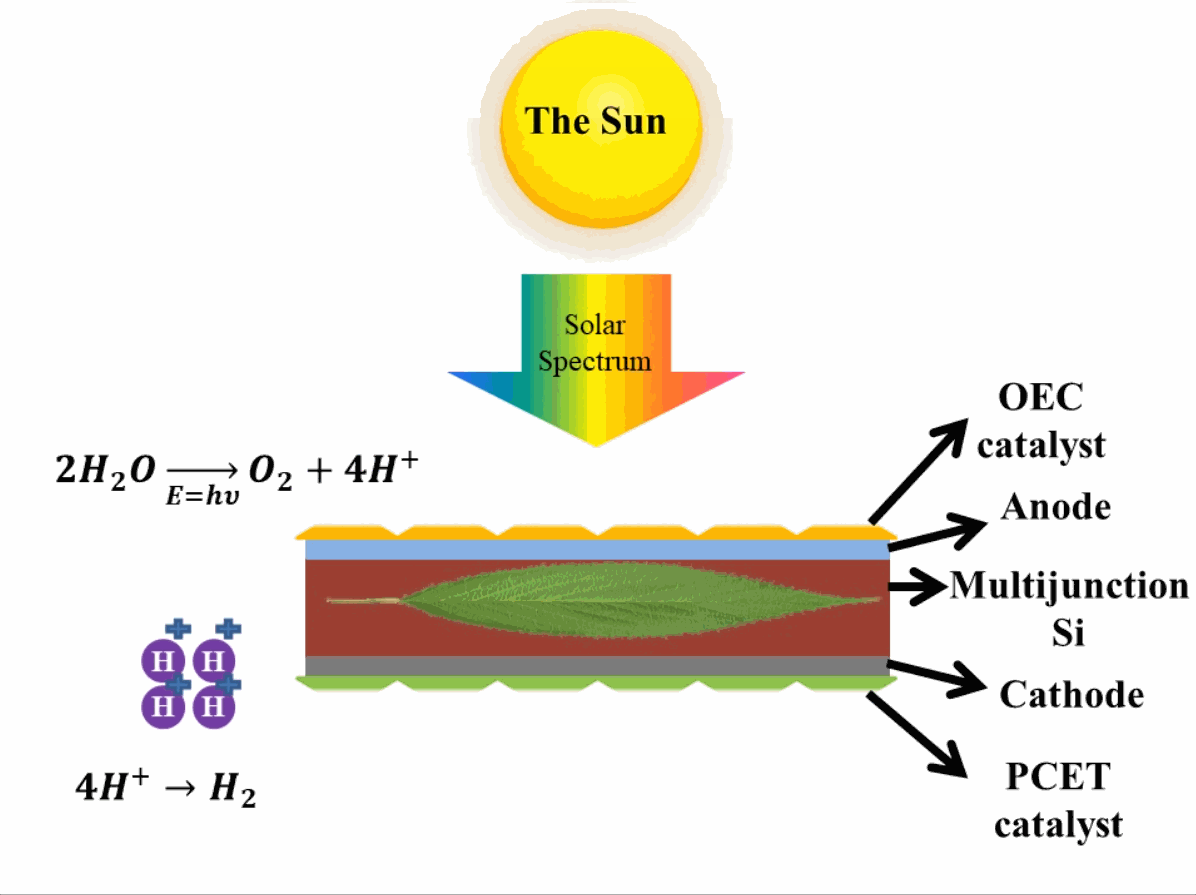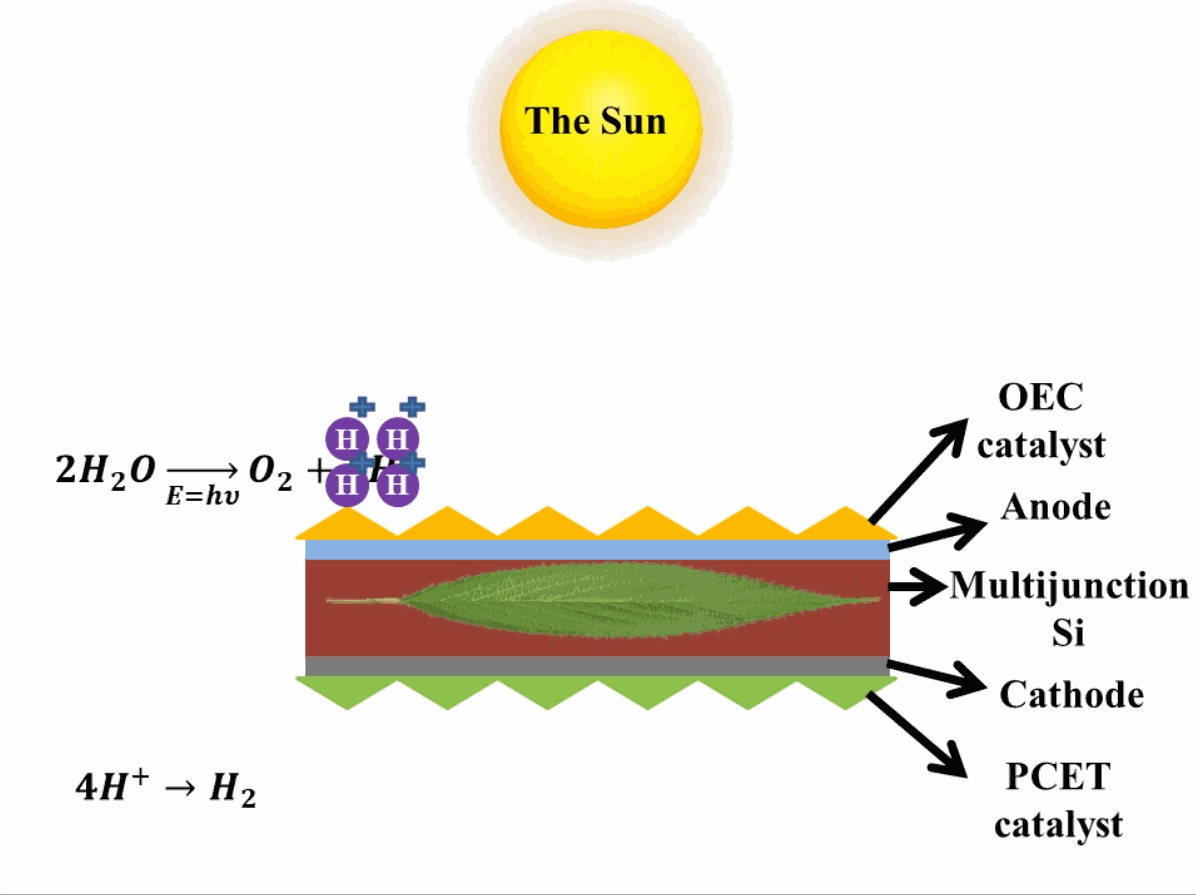
Advantage of artificial leaf systems
- It can store energy in chemical form, just like a battery unlike solar panels that require external batteries.
- All the byproducts of this system are environment friendly.
- It is mad from materials that are abundant on earth.
- It has a potential to capture carbon, as well as producing fuels. Even if fuels are used, it is still a carbon-neutral process of extracting solar energy.
- Can be an artificial substitute to plants and trees and help combat climate chage and reduce global warming.
Disadvantages
- Materials of anode and cathode corrode in water.
- It is not yet cost-effective to replace the solar cell technology.
- It has low efficiency in terms of fuel generation.
References and Further readings
- Ciamician, G. (1912). The photochemistry of the future. Science, 36(926), 385-394.
- Gan, P., Liu, F., Li, R., Wang, S., & Luo, J. (2019). Chloroplasts—beyond energy capture and carbon fixation: tuning of photosynthesis in response to chilling stress. International journal of molecular sciences, 20(20), 5046.
- Nocera, D. G. (2012). The artificial leaf. Accounts of chemical research, 45(5), 767-776.
- Michl, J. (2011). Towards an artificial leaf?. Nature chemistry, 3(4), 268-269.
- Wu, Y. A., McNulty, I., Liu, C., Lau, K. C., Liu, Q., Paulikas, A. P., … & Rajh, T. (2019). Facet-dependent active sites of a single Cu 2 O particle photocatalyst for CO 2 reduction to methanol. Nature Energy, 4(11), 957-968.
