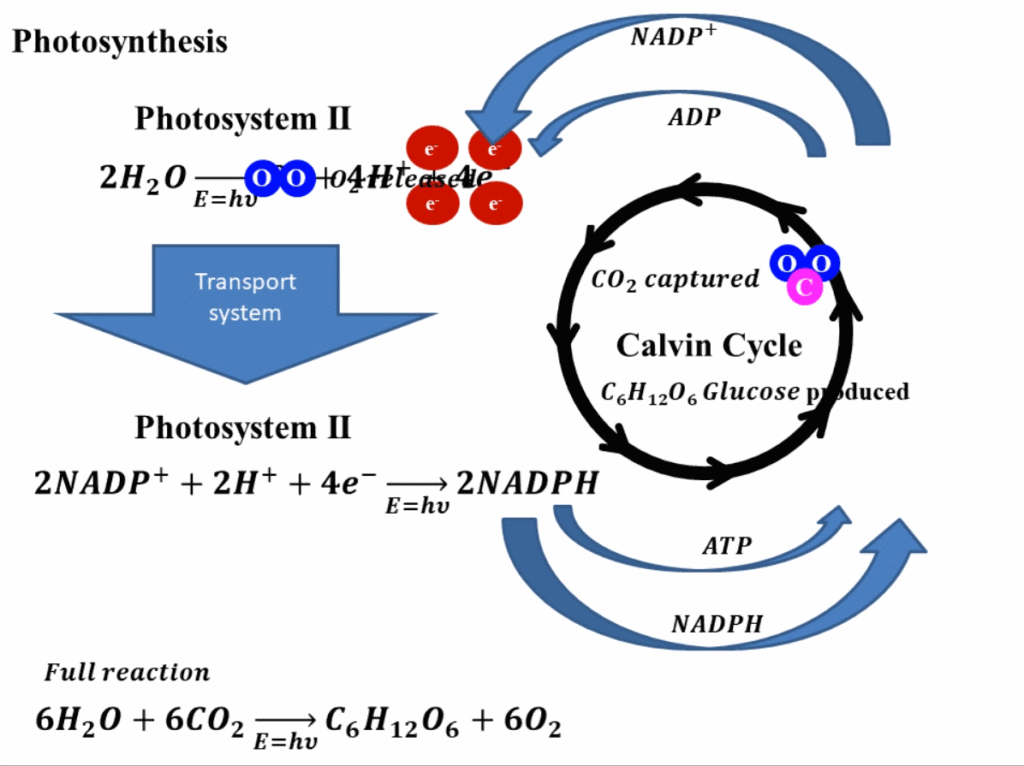
Perovskite solar cells
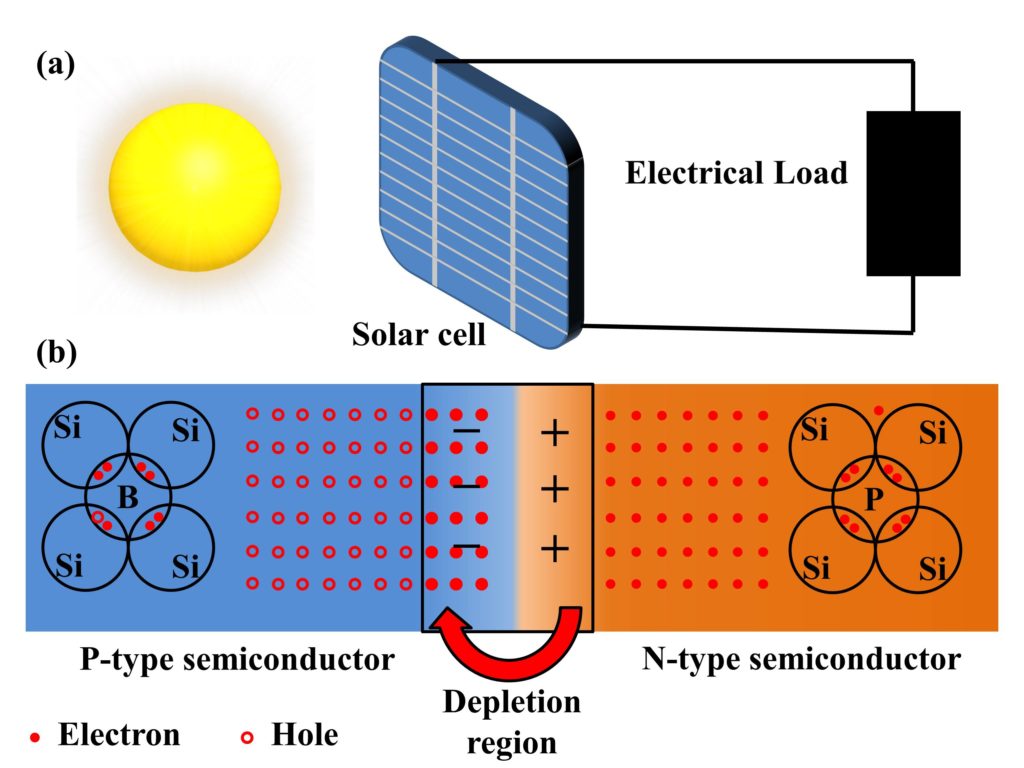
A perovskite solar cell is a solar energy harvesting cell, just like other technologies of the solar cells. However, as its name suggests, perovskite solar cells have an active perovskite material whose structure of crystal resembles the crystal structure of calcium titanium oxide (CaTiO3). All perovskite compounds have an inherent structure of ABX3, where both A and B are cations with an oxidation state of +1 and +2, and X is an anion that bonds to both cations having a usual oxidation state of -1. The usual elements that are used for A place are methylammonium (CH3NH3+), Caesium (Cs+), and Rubidium (Rb+). The usual elements for B cation are Lead (Pb2+), Tin (Sn2+), and Germanium (Ge2+). For anions at position X, usually, halides are used and amongst them, most commonly Iodide (I–), Chloride (Cl–), Bromide (Br–) anions are used.
Construction of perovskite solar cells
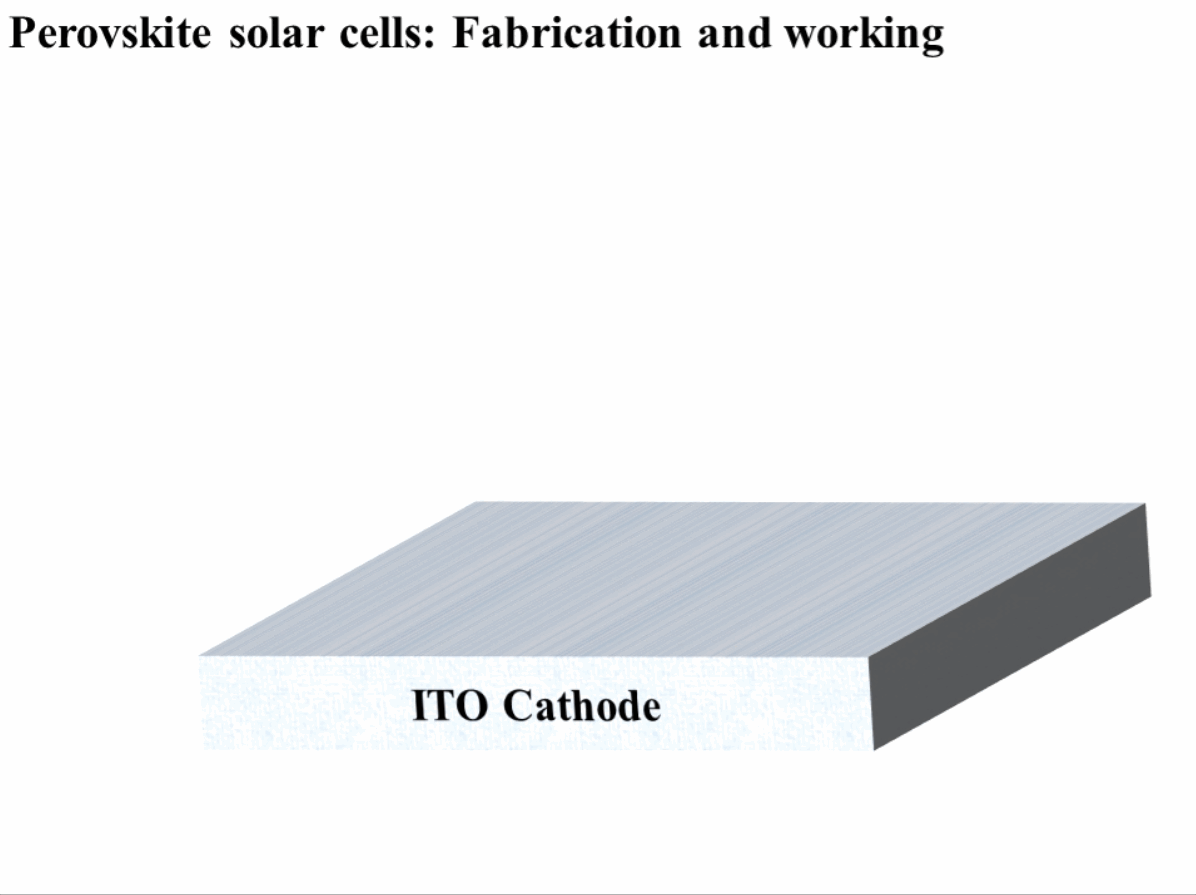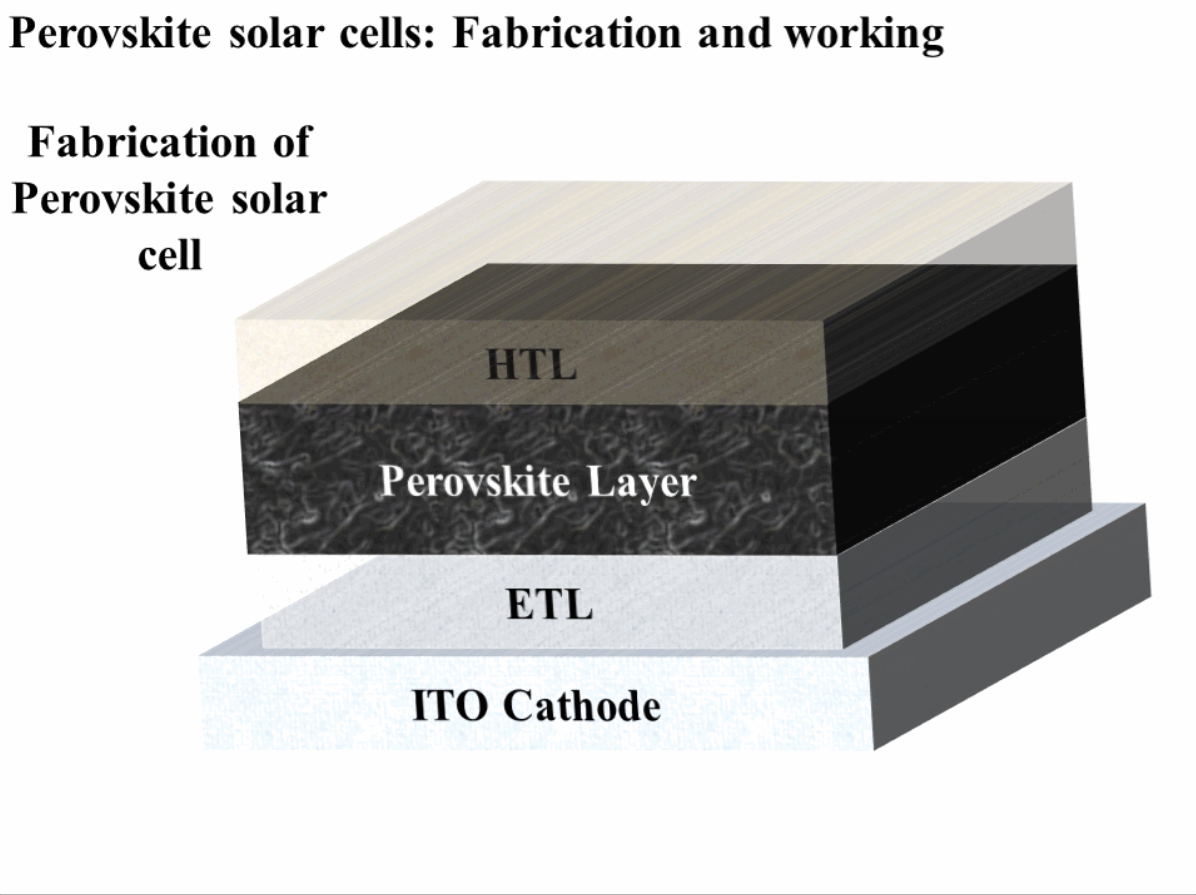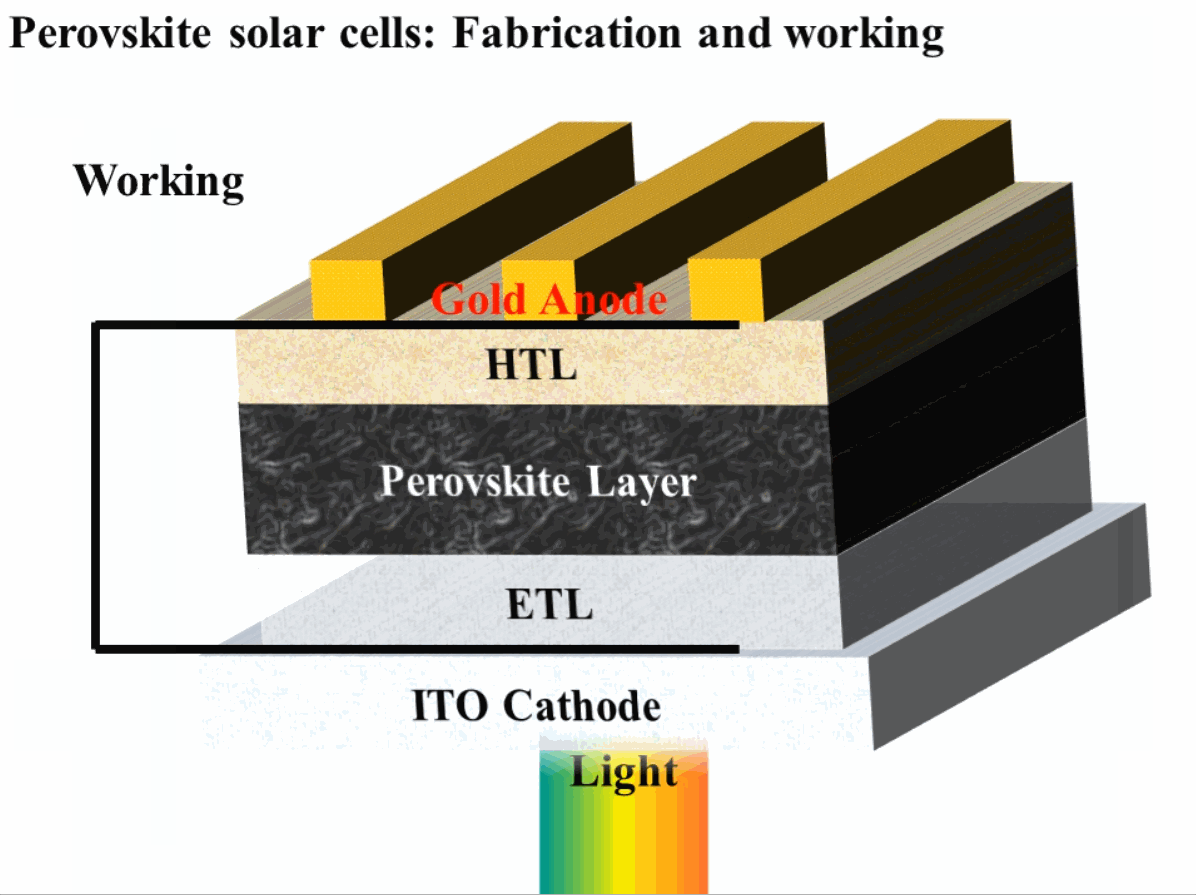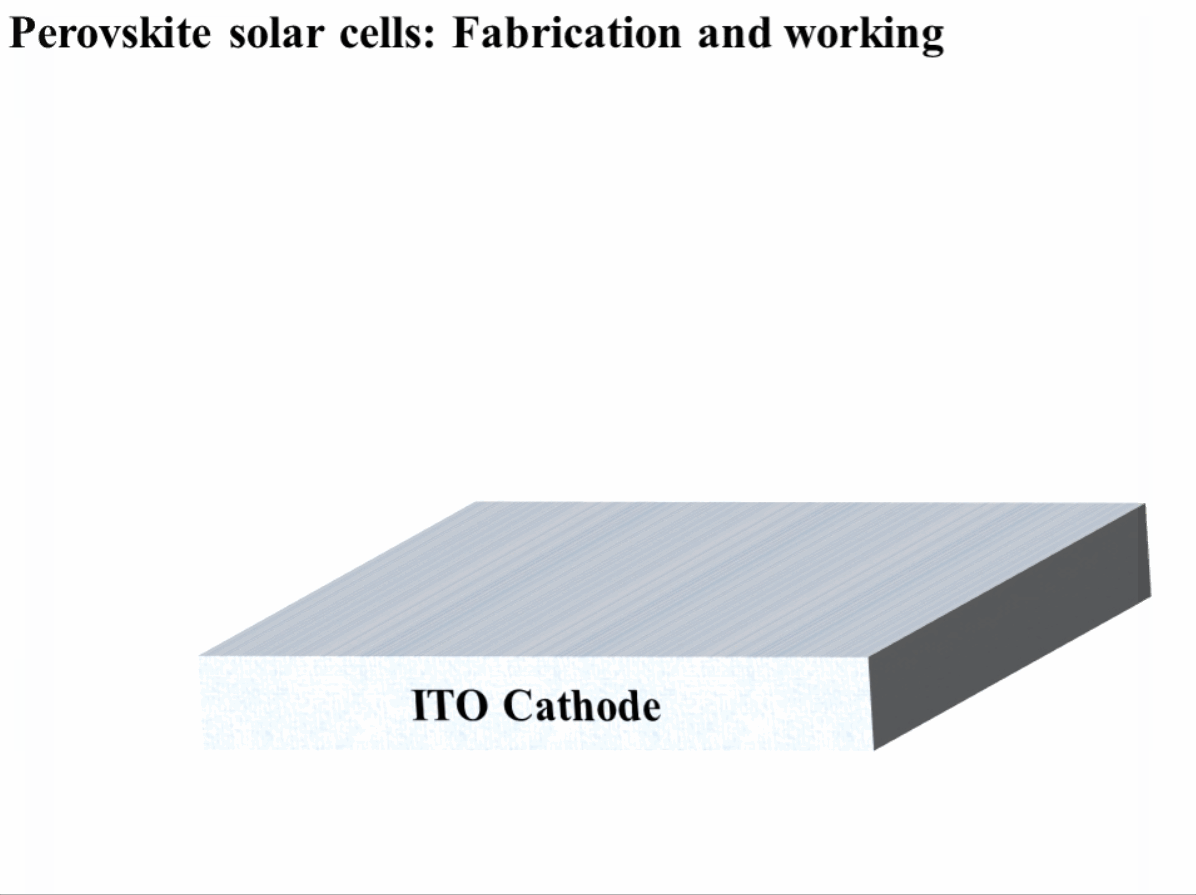
The construction of perovskite solar cells (PSC) requires an active perovskite material such as methylammonium lead iodide (CH3NH3PbI3). The PSC solar cells are usually fabricated with the top-down fabrication method. The major steps involving fabrication and working are mentioned in the sequence below.
- Transparent cathode: A transparent cathode of glass (SiO2) chosen such that it has a coating of a very thin conductive layer on one side and the other side of the glass is an insulator. Usually Flourine-doped Tin Oxide (FTO) is used for the conduction side of the glass. The major purpose of this transparent conduction layer is to allow the transmission of light from one side of the perovskite solar cells.
- Electron Transport Layer (ETL): The electron transport layer is coated on top of the FTO layer, and its major purpose is to transport electrons to the cathode. It also serves as a layer to stop holes. Usually titanium-dioxide (TiO2) is used as ETL in PSCs. Since the light also needs to be transmitted from this side, a careful material selection is required, which needs to not only transport electrons but also be transparent such as TiO2.
- Perovskite layer: Perovskite material layer is used to absorb the sunlight and generate electron-hole pairs which are carried to the cathode and anode in order to generate electric current, and therefore, electric power. The perovskite material layer is usually spin-coated on electron transport layer, and after coating, this layer is heated where it turns into a black layer.
- Hole Transport Layer (HTL): The hole transport layer (HTL) is coated on top of the pervoskite layer. The purpose of HTL is opposite to that of ETL, where this layer is used to transport holes and block the flow of electrons.
- Anode: The anode is made up of highly conductive metal and it used to conduct holes to the external circuit for the production of electric power. Usually Gold (Au) is used as an anode material because of its high conductivity. Patterned anodes can be made to increase the efficiency of hole collection, which helps to yield higher efficiency of perovskite solar cells.
The step-by-step fabrication and working of a PSC are shown in Fig. 1.
Future of perovskite solar cells
Currently the latest perovskite solar cells have crossed 25.5% efficiency, whereas monolithic tandem Si perovskite solar cells have crossed 29.5% efficiency. These efficiencies are much higher or comparable to single junction, monocrystalline Si solar cells. The detailed advantages and disadvantages of employed perovskite solar cells are written below.
Advantages
- The active perovskite materials provide a direct bandgap of around 1.5 eV.
- Due to higher bandgap, they can effectively absorb ultraviolet spectrum of the solar energy and therefore can work effectively in tandem solar cells.
- They are easy to fabricate, and sometimes do not involve lithography.
- The materials required in perovskites are relatively cheap in comparison to Si-based solar cells.
- They can achieve astonishingly high efficiencies, in comparison to low complexities involved in their fabrication.
Disadvantage
- The active perovskite materials deteriorate continuously in environment, making perovskite solar cells to have a low-life.
- Perovskite materials can become useless when exposed to air or moisture.
- The efficiency of PSC depend upon the quality of coated thin films. If coating is poor, they efficiency can decrease significantly.
- If lead is used as one of the cations, the fabrication of PSC can also have safety concerns as lead is poisonous.
Concluding remarks: The perovskite solar cell is a very neat technology with high efficiency and it definitely has a future, but more research into its longevity is required before it can be employed industrially.
Further readings
If you liked this post, you may be interested in reading the following closely related topics.